The bond angle for the C-N-O bonds in the following molecule would be expected to be approximately: 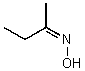
A) 90
B) 109
C) 120
D) 145
E) 180
Correct Answer:
Verified
Q124: Which molecule would be linear? (In each
Q125: The bond angle for the C-C-O
Q126: The bond angle for the C-P-C
Q127: All organic compounds have their origins from
Q128: The bond angle for the C-S-C
Q130: The major resonance contributor to the resonance
Q131: Contributing resonance structures cannot have contributors whose
Q132: VSEPR theory predicts an identical shape for
Q133: Which of the following would have a
Q134: The bond angle for the C-C-N
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents