Which molecule(s) has/have dipole moment(s) equal to zero?
A) 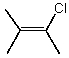
B) 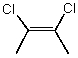
C) 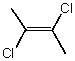
D) 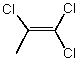
E) None of these choices have dipole moments equal to zero.
Correct Answer:
Verified
Q5: What alkyl group is attached to the
Q6: What alkyl groups make up the following
Q7: Which molecule has a dipole moment of
Q8: Which molecule has a zero dipole moment?
A)CO2
B)CH4
C)CH3CH3
D)
Q9: Of the following solvents which one does
Q11: What alkyl groups make up the following
Q12: What alkyl groups are attached to the
Q13: Which molecule has a zero dipole moment?
A)SO2
B)CO2
C)CO
D)CHCl3
E)None
Q14: Of the following common organic solvents which
Q15: Which molecule has a zero dipole moment?
A)CH3Cl
B)CH2Cl2
C)CHCl3
D)CCl4
E)None
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents