The following substance is expected to have the lowest solubility in which of the following solvent(s) ? 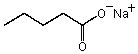
A) CCl4
B) C2H5OH
C) CHCl3
D) HOCH2CH2CH2CH2CH2CH2OH
E) The given substance is likely to be quite soluble in all of the solvents described.
Correct Answer:
Verified
Q83: For the functional group(s)on the following molecule,what
Q84: Which compound would have the lowest boiling
Q85: The compound NaOH is barely soluble in
Q86: For the functional group(s)on the following molecule
Q87: For the functional group(s)on the following molecule,what
Q89: The IR spectrum of which type of
Q90: An oxygen-containing compound shows strong IR absorption
Q91: The solid alkane CH3(CH2)18CH3 is expected to
Q92: The absorption band for the O-H stretch
Q93: For the functional group(s)on the following molecule,what
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents