Rank the following hydrogen atoms from highest to lowest in acidity in the structure shown below. 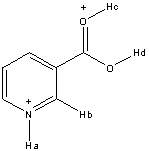
A) Ha > Hb > Hc > Hd
B) Ha > Hc > Hd > Hb
C) Hc > Hd > Ha > Hb
D) Hd > Ha > Hc > Hb
E) Hc > Ha > Hd > Hb
Correct Answer:
Verified
Q30: Which is the strongest acid?
A)CH3CH2OH
B)CH3CO2H
C)HC
Q31: The reaction between which combination of substances
Q32: For the equilibrium Q33: Rank the bold-faced hydrogens for the following Q34: Rank the bold-faced hydrogens for the following Q36: The acidity constant,Ka,differs from the equilibrium constant,Keq,for Q37: Hydrogen atom(s)from which position(s)is (are)most likely to Q38: Rank the bold-faced hydrogens for the following Q39: Which of these is not a diprotic Q40: Which of the following substances has a![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents