For the following acid/base reaction which statement is true taking S into consideration? 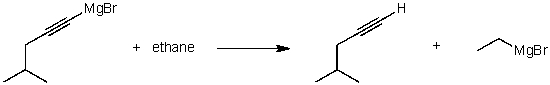
A) The reaction is an exothermic reaction and S is approximately zero.
B) The reaction is an endothermic reaction and S is negative.
C) The reaction is an exothermic reaction and S is negative.
D) The reaction is an exothermic reaction and S is positive.
E) None of these choices.
Correct Answer:
Verified
Q58: What prediction can be made of the
Q59: For the simple hydrides,MHn,pKa values decrease in
Q60: An acid,HA,has the following thermodynamic values
Q61: For the following acid-base reaction,which statement
Q62: Which is the weakest acid?
A)CH3CH2CH2CH2CHFCO2H
B)CH3CHCH2CH2CH2CH2OH
C)CH3CH2CH2CH2CH2SO3H
D)CH3CH2CH2CH2CH=CH2
E)CH3CH2CH2CH2NH2
Q64: For the following acid / base
Q65: For the following acid/base reaction which
Q66: Rank the bold-faced hydrogens for the following
Q67: For the following acid-base reaction,which statement
Q68: For the following acid / base
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents