For the following acid / base reaction which statement is true taking H into consideration? 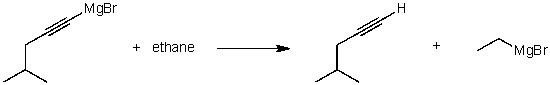
A) The reaction is an exothermic reaction and H is approximately zero.
B) The reaction is an endothermic reaction and H is negative.
C) The reaction is an exothermic reaction and H is negative.
D) The reaction is an exothermic reaction and H is positive.
E) None of these choices.
Correct Answer:
Verified
Q75: For the following acid-base reaction,which statement
Q76: For the following acid/base reaction which
Q77: Which is the strongest acid?
A)CH3CH2CH2CH2CHFCO2H
B)CH3CHBrCH2CH2CH2CO2H
C)CH3CH2CH2CHClCH2CO2H
D)CH3CH2CH2CHFCH2CO2H
E)CH3CH2CH2CHICH2CO2H
Q78: Which of the following is an
Q79: For the following acid/base reaction which
Q81: Which base would not effectively deprotonate acetylene?
A)i-PrMgBr
B)KH
C)CH3OCH2Li
D)(i-Pr)2NLi
E)t-BuOK
Q82: Acetic acid dissociates most completely in:
A)CCl4
B)Cl2C=CCl2
C)H2O
D)(CH3CH2)2O
E)the gas
Q83: What compounds are produced when NaSH is
Q84: As a consequence of the "leveling effect,"
Q85: Which acid-base reaction would not take
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents