The isomer of alanine shown below is one of the 20 naturally occurring amino acids that are used to make proteins.Amino acids like alanine exist at neutral acidity (pH = 7)in the following form: 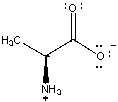 What would be the structure of alanine if HCl(aq)was added to lower the pH = 1? What would be the structure of alanine if NaOH(aq)was added until the pH = 12?
What would be the structure of alanine if HCl(aq)was added to lower the pH = 1? What would be the structure of alanine if NaOH(aq)was added until the pH = 12?
Correct Answer:
Verified
Q119: Which reaction of these potential acids and
Q120: Which combination of reagents is effective in
Q121: Why do water-insoluble carboxylic acids dissolve in
Q122: Reagents that seek to react with an
Q123: Protons or other electron-deficient centers that seek
Q125: The strongest acid that can exist in
Q126: According to Lewis theory,a base is a
Q127: For a reaction that has positive change
Q128: It is very difficult to deprotonate a
Q129: Which of the reaction conditions could afford
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents