If the following compound were treated with excess NaOH followed by an excess of D2O,what would be the structure of the new product formed? 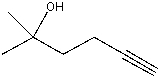
Correct Answer:
Verified
Q137: In order for an acid-base reaction to
Q138: What is the major product for the
Q139: Write an equation to show the reaction
Q140: According to Bronsted-Lowry theory,an acid is a
Q141: Briefly,but clearly,explain why the -OH hydrogen in
Q143: You are planning to carry out a
Q144: Define a protic solvent.
Q145: Isotope labeling is an important tool in
Q146: You are planning to purify an impure
Q147: What are the two fundamental types of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents