The most stable conformation for 1,2-ethanediol (ethylene glycol) is shown below.It is the most stable conformation because 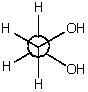
A) this corresponds to an anti conformation.
B) in general,gauche conformations possess the minimum energy.
C) it is stabilized by intramolecular hydrogen bonding.
D) it is a staggered conformation.
E) it has the highest energy of all the possibilities.
Correct Answer:
Verified
Q56: The IUPAC name for diisobutylacetylene is
A)2,7-Dimethyl-4-octene
B)2,7-Dimethyl-4-octyne
C)3,6-Dimethyl-4-octyne
D)2,5-Diethyl-3-hexyne
E)2,2,5,5-Tetramethyl-3-hexyne
Q57: The graph below is a plot of
Q58: Which conformation(s)show(s)the bromines in a gauche orientation
Q59: The most stable conformation of 3-bromo-2-methylpentane,viewed through
Q60: Give the IUPAC name for 
Q62: cis-1,2-Dibromocyclohexane is represented by structure(s): 
Q63: The least stable conformation of 2,3-dimethylpentane,viewed through
Q64: Select the systematic name for 
Q65: Which cycloalkane has the least ring strain?
A)Cyclopropane
B)Cyclobutane
C)Cyclopentane
D)Cyclohexane
E)Cycloheptane
Q66: What structure represents the most stable conformation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents