The most stable conformation of 3-bromo-2-methylpentane,viewed through the C-2-C-3 bond (i.e.,C-2 in the front,C-3 in the back) : 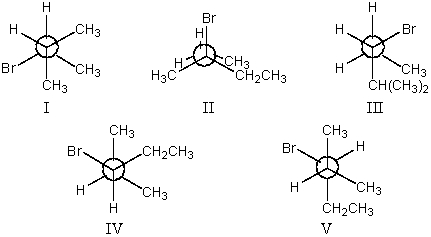
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q65: Which cycloalkane has the least ring strain?
A)Cyclopropane
B)Cyclobutane
C)Cyclopentane
D)Cyclohexane
E)Cycloheptane
Q66: What structure represents the most stable conformation
Q67: trans-1,2-Dibromocyclohexane is represented by structure(s): 
Q68: The preferred conformation of cis-3-tert-butyl-1-methylcyclohexane is the
Q69: Which is the most stable conformation of
Q71: The graph below is a plot of
Q72: The twist boat conformation is the preferred
Q73: What is the most thermodynamically stable chair
Q74: Which cycloalkane has the greatest ring strain?
A)Cyclopropane
B)Cyclobutane
C)Cyclopentane
D)Cyclohexane
E)Cycloheptane
Q75: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents