The compounds whose structures are shown below would have: 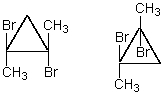
A) the same melting point.
B) different melting points.
C) equal but opposite optical rotations.
D) More than one of these choices.
E) None of these choices.
Correct Answer:
Verified
Q91: I and II are: Q92: Which molecule has a plane of symmetry? Q93: The two compounds shown below are: Q94: The compounds whose molecules are shown below Q95: How many different compounds are there which Q97: Which molecule is a meso compound? Q98: I and II are: Q99: Which one of the following can exist Q100: I and II are: Q101: I and II are: Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()

