Consider the SN2 reaction of 1-chloro-5-methylhexane with CN- ion. 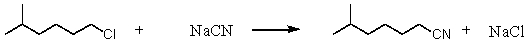
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 1-chloro-5-methylhexane and NaCN?
A) No effect.
B) It would double the rate.
C) It would triple the rate.
D) It would increase the rate four times.
E) It would increase the rate six times.
Correct Answer:
Verified
Q15: Select the potential energy diagram that represents
Q16: Increasing the temperature of a chemical reaction
Q17: What product(s)would you expect to obtain from
Q18: Select the rate law for the
Q19: Select the potential energy diagram that represents
Q21: Ambident nucleophiles are ones which can react
Q22: Rank the following in terms of nucleophilic
Q23: Which is the weakest nucleophile in polar
Q24: An increase in the kinetic energy of
Q25: Reaction of (R)-2-chloro-4-methylhexane with excess NaI in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents