The reaction, 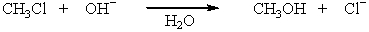 has the following thermodynamic values at 27.0 ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
has the following thermodynamic values at 27.0 ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
A) -73.8 kJ mol-1
B) -76.8 kJ mol-1
C) -59.0 kJ mol-1
D) +91.6 kJ mol-1
E) -91.6 kJ mol-1
Correct Answer:
Verified
Q1: An increase in the temperature at which
Q2: Select the potential energy diagram that represents
Q3: Consider the SN2 reaction of butyl
Q4: The rate equation for a nucleophilic substitution
Q5: Consider the SN2 reaction of 2-iodopentane with
Q7: If the rate of reaction of [0.1
Q8: The difference in the bond energies
Q9: The hybridization state of the charged carbon
Q10: The rate equation for a nucleophilic substitution
Q11: Which will be true for any
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents