When diastereomers I and II undergo an E2 elimination on treatment with sodium ethoxide in ethanol,one of the isomers react 500 times faster than the other one.Also,one isomer gives only A as a product and the other isomer gives a mixture of A and B as products.Determine the products of each isomer and explain your reasoning. 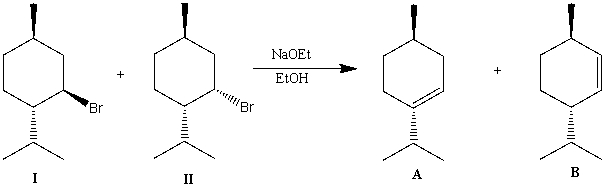
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q203: What would be the major product(s)of the
Q204: All SN1 reactions will always be accompanied
Q205: The product(s)for the following reaction would mainly
Q206: Predict the major product(s)for the following reaction
Q207: The product(s)for the following reaction would mainly
Q209: What would be the major product(s)of the
Q210: Which would be the major product of
Q211: Predict the product(s)for the following reaction sequence.
Q212: Heating tert-butyl chloride with 1.0 M NaOH
Q213: What would be the major product(s)of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents