Which compound below would not give rise to 4 signals in the proton NMR spectrum and 3 signals in the carbon NMR spectrum? (Assume you can separate and see all peaks.) 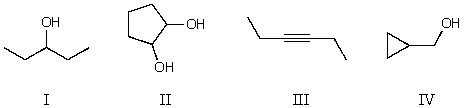
A) I
B) II
C) III
D) IV
E) All of these choices fit the criteria.
Correct Answer:
Verified
Q111: What is the structure of the compound
Q112: What is the structure of the compound
Q113: An organic compound absorbs strongly in the
Q114: Which is the base peak? 
Q115: What is the structure of the compound
Q117: For the following compound how many different
Q118: A bromodichlorobenzene which gives four signals in
Q119: Predict the base peak for 2-chloro-2-methylpropane,
A)m/z 15
B)m/z
Q120: For the following compound how many different
Q121: Predict the 1H NMR spectrum of 2-chloroethanal,CH2ClCHO.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents