Which free radical would be least stable? 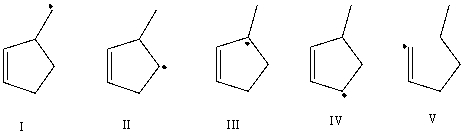
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q75: Consider the light-initiated chlorination of (S)-2-chlorobutane followed
Q76: What would be the major product of
Q77: Which of the following would be the
Q78: Which hydrogen atom(s)of Q79: Which free radical would be most stable? Q81: 2-Methyl-2-butene reacts with HBr in the presence Q82: What product would result from the following Q83: What would be the major product of Q84: What would be the major product of Q85: Which of the following combinations of reactants![]()
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents