Which hydrogen atom(s) of 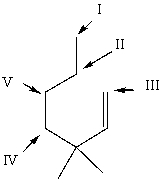 is/are least susceptible to abstraction by free radicals?
is/are least susceptible to abstraction by free radicals?
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q62: Which free radical would be least stable?
A)
Q63: Which carbon of Q64: Which hydrogen atom(s)of Q65: What would be the major product of Q66: Which free radical would be most stable? Q68: Which of the following would be the Q69: Which of the following compounds is not Q70: Which free radical would be most stable? Q71: The free radical chlorination of 3-chloropentane forms Q72: An unsaturated product results from the![]()
![]()
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents