CrO3 in H2SO4/H2O will fail to give a positive test with which of these compounds?
A) CH3CH2CH2CH2OH
B) 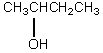
C) (CH3) 3COH
D) 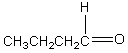
E) More than one of these choices.
Correct Answer:
Verified
Q119: What would be the major product of
Q120: What would be the major product of
Q121: Which of the reagents listed below would
Q122: Fundamentally,2-methyl-2-pentanol does not undergo oxidation by H2CrO4
Q123: Which of these is the least reactive
Q125: When 2-pentanol is treated with chromic acid,
A)the
Q126: Which of the reagents listed below would
Q127: If the role of the solvent is
Q128: Jones reagent will fail to give a
Q129: Which of the reagents/techniques listed below would
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents