The accompanying diagram,which describes the fate of the intermediate in a reversible reaction,implies that: 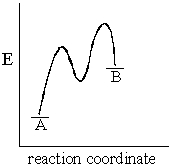
A) the less stable product forms more rapidly.
B) the more stable product forms more rapidly.
C) product B will predominate at equilibrium.
D) the intermediate has a short lifetime.
E) No conclusions can be drawn as to either reaction rate or product stability.
Correct Answer:
Verified
Q84: Indicate which products would be obtained
Q85: Which is an untrue statement concerning the
Q86: Which reagent would convert 1,3-pentadiene into 3-penten-2-ol?
A)KMnO4/-OH
B)OsO4
C)H2O2,then
Q87: Ignoring stereochemistry,the 1:1 reaction of bromine
Q88: Select the most energetically favorable UV
Q90: Which reagent would convert 1,3-octadiene into 3-octen-2-ol?
A)KMnO4/-OH
B)OsO4
C)H2O2,then
Q91: Which is the only compound which can
Q92: The accompanying diagram implies that: 
Q93: Ignoring stereochemistry,the 1:1 reaction of chlorine
Q94: A reaction under kinetic (or rate)control will
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents