The accompanying diagram,which describes the fate of the intermediate in a reversible reaction,implies that: 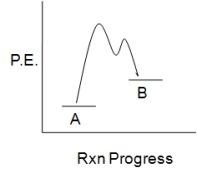
A) the less stable product forms more rapidly.
B) the more stable product forms more rapidly.
C) product B will predominate at equilibrium.
D) the intermediate has a short lifetime.
E) No conclusions can be drawn as to either reaction rate or product stability.
Correct Answer:
Verified
Q93: Ignoring stereochemistry,the 1:1 reaction of chlorine
Q94: A reaction under kinetic (or rate)control will
Q95: How could the following synthesis be carried
Q96: An equilibrium-controlled reaction will yield predominantly:
A)the more/most
Q97: Which compound would have a UV absorption
Q99: A thermodynamically-controlled reaction will yield predominantly:
A)the more/most
Q100: Keeping in mind stereochemistry,how many products are
Q101: Which of the following can undergo the
Q102: From the standpoint of reactivity,which is the
Q103: Which of the following pairs of compounds
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents