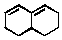
 does not undergo the Diels-Alder reaction as a diene because:
does not undergo the Diels-Alder reaction as a diene because:
A) a bicyclic ring system cannot function as the diene component.
B) it cannot adopt the s-cis conformation.
C) it lacks electron-withdrawing groups.
D) it lacks strong electron-releasing groups.
E) the two double bonds are further apart than in a non-cyclic conjugated system.
Correct Answer:
Verified
Q130: Q131: Which of the following would afford a Q132: Which diene would be least reactive toward Q133: Which of the following dieneophiles is most Q134: What would be the product of the Q136: Which diene and dienophile would you choose Q137: Which of the following would afford a Q138: Which of the following would afford a Q139: Which diene would you expect to react Q140: Which of the following dieneophiles is most![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents