A compound has the formula C8H9Br.Its 1H NMR spectrum consists of: doublet, 2.0
Quartet, 5.15
Multiplet, 7.35
The IR spectrum shows two peaks in the 680-840 cm-1 region; one is between 690 and 710 cm-1 and the other is between 730 and 770 cm-1.Which is a possible structure for the compound? 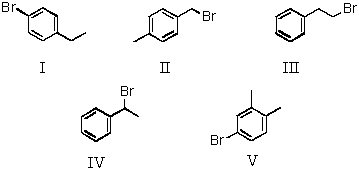
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q136: How many resonances would be expected in
Q137: Which of the following would you expect
Q138: How many resonances would be expected in
Q139: How many equivalent resonance structures can be
Q140: How many equivalent resonance structures can be
Q142: The difference between the amount of heat
Q143: Which isomer of C7H7Cl exhibits strong IR
Q144: Which of the following substances,C8H9Cl,would exhibit five
Q145: Cagelike molecules with the geometry of a
Q146: How many resonances would be expected in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents