Briefly explain why the aromatic hydrocarbon shown possesses a significant dipole moment.Use diagrams as needed to illustrate/clarify your answer. 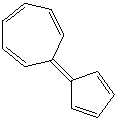
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q165: When a benzene ring is used as
Q166: The heats of hydrogenation for cyclohexene
Q167: Draw the structure corresponding to the following
Q168: Explain briefly why cyclopentadiene readily reacts with
Q169: Hückel's Rule requires _
Q171: The pKa of cyclopentadiene is found to
Q172: Benzene,while unusually unreactive with electrophiles,will react under
Q173: Pyrrole is not particularly basic,because the lone
Q174: Use the "polygon-in-circle" method to draw an
Q175: Predict the product of the reaction of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents