Which of the following contributors to the resonance stabilized hybrid formed when aniline undergoes para-chlorination would be exceptionally stable? 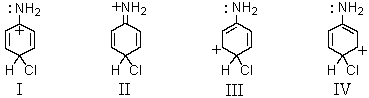
A) I
B) II
C) III
D) IV
E) None of these choices.
Correct Answer:
Verified
Q15: Which of these is the rate-determining step
Q16: Which of the following structures contribute(s)to the
Q17: The electrophilic bromination or chlorination of benzene
Q18: Consider the structures given below.Which of them
Q19: Which of the following structures contribute(s)to the
Q21: Which of the following reactions would produce
Q22: Which of these is a satisfactory
Q23: Which of the following reactions would yield
Q24: Which of the following reactions could be
Q25: Which reagent or test could you use
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents