The correct structure for ethyl 3-methylbutanoate is: 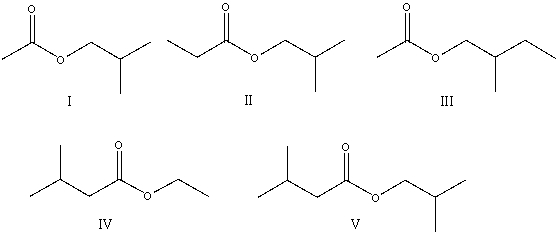
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q6: Which compound would be the strongest acid?
A)4,4-dichlorobutanoic
Q7: Which of the following is the best
Q8: The correct structure for bicyclo[1.1.1]pentane-2-carboxylic acid is:
Q9: What is the IUPAC name for
Q10: The correct structure for bicyclo[2.2.2]octane-2-carboxylic acid is:
Q12: Which of the following structures is 3,4-dimethylpentyl
Q13: The correct structure for bicyclo[1.1.0]butane-2-carboxylic acid is:
Q14: The correct structure for ethyl 3-methylbutanoate is:
Q15: The correct structure for ethyl 2-chloropentanoyl chloride
Q16: Which compound would be the weakest acid?
A)CHCl2CH2CH2CO2H
B)ClCH2CHClCH2CO2H
C)CH3CCl2CH2CO2H
D)CH3CHClCHClCO2H
E)CH3CH2CCl2CO2H
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents