The pKa of benzoic acid is 4.20.A benzoic acid substituted in the para-position with some group G (shown below)is reported to have a pKa of 3.92. 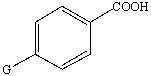 Based on this information is group G an electron withdrawing or electron donating group?
Based on this information is group G an electron withdrawing or electron donating group?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q199: The linkages that join amino acids together
Q200: The only carboxylic acid derivative with two
Q201: Complete the following reaction sequence,giving details of
Q202: Complete the following reaction sequence,giving details of
Q203: Carbonation of a Grignard reagent or the
Q205: Carbonation of a Grignard reagent or the
Q206: When a gamma-hydroxy acid is treated with
Q207: Complete the following reaction sequence,giving details of
Q208: Carbonation of a Grignard reagent or the
Q209: Suggest a suitable synthetic strategy for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents