Compound W has the molecular formula C11H17N.Treatment of W with benzenesulfonyl chloride in base gives no reaction.Acidification of the resulting mixture gives a clear solution.The 1H NMR spectrum of W consists of: triplet, 1.0
Quartet, 2.5
Singlet, 3.6 (2H)
Multiplet, 7.3 (5H)
The most likely structure for W is:
A) 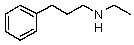
B) 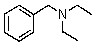
C) 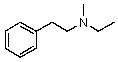
D) 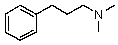
E) 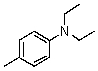
Correct Answer:
Verified
Q17: What type of amine is N-methyl-2-methyl-3-hexanamine?
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of
Q18: Which of the following is a tertiary
Q19: The aromatic amine imidazole contains how many
Q20: What type of amine is pyrrolidine?
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of
Q21: Which of these alkyl halides cannot be
Q23: Which reagent will distinguish between 2-amino-2,3-dimethylpentane and
Q24: Which reagent would serve as the basis
Q25: The reaction of which of these compounds
Q26: This type of compound is the
Q27: The correct structure of (R)-N-methylbicyclo[2.2.1]heptan-2-amine is?
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents