Answer the question(s) below about the following reaction.
2 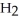
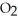 → 2
→ 2 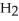 O +
O + 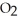
-How many grams of hydrogen peroxide are needed to produce 25.0 g of oxygen?
A) 106 g
B) 26.6 g
C) 5.88 g
D) 25.0 g
E) 53.2 g
Correct Answer:
Verified
Q87: The number of particles in 1 mole
Q88: Acetylene gas, C2H2 ,reacts with oxygen
Q89: The molar mass of magnesium is 24.31
Q90: The moles of NH3 in 55.5 g
Q91: What type of reaction is the following?
2KClO3
Q93: Avogadro's number is the number of grams
Q94: What is the mass,in grams,of 1.00 mole
Q95: When 25.0 g of KClO3 reacts,how many
Q96: The molar mass of silver is 47
Q97: Answer the question(s)below about the following reaction.
2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents