The compound 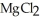 is named ________.
is named ________.
A) magnesium chlorine
B) magnesium dichloride
C) magnesium(II) chloride
D) magnesium chloride
E) dimagnesium chloride
Correct Answer:
Verified
Q2: An ionic compound _.
A)has a net positive
Q3: In ionic compounds,_ lose their valence electrons
Q4: Elements in group 2A (2)of the periodic
Q5: To form an ion,a sodium atom _.
A)gains
Q6: What is the ionic charge of an
Q8: What is the symbol for the ion
Q9: An anion always _.
A)has a positive charge
B)contains
Q10: How many electrons will aluminum gain or
Q11: What is the correct formula for the
Q12: The ion of aluminum is _.
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents