The bond in 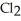 is a(n) ________.
is a(n) ________.
A) ionic bond
B) nonpolar covalent bond
C) metallic bond
D) polar ionic bond
E) no bond
Correct Answer:
Verified
Q42: The ability of an atom to attract
Q43: Ionic bonding is expected in which of
Q44: The ammonia molecule (NH3)is _.
A)a polar molecule
Q45: The shape of the ammonia molecule (NH3)is
Q46: The shape of the water molecule (H2O)is
Q48: The main type of intermolecular forces between
Q49: The main type of intermolecular forces between
Q50: The carbon tetrachloride molecule,CC Q51: Double and triple bonds form because _. Q52: A polar covalent bond is found in![]()
A)the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents