The specific heat of copper is 0.0920 cal/g °C,and the specific heat of silver is 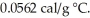 If 100 cal of heat is added to one g of each metal at 25 °C,what is the expected result?
If 100 cal of heat is added to one g of each metal at 25 °C,what is the expected result?
A) The copper will reach a higher temperature.
B) The silver will reach a higher temperature.
C) The two samples will reach the same temperature.
D) The copper will reach a temperature lower than 25 °C.
E) The silver will soften.
Correct Answer:
Verified
Q54: The physical state(s)present when a substance is
Q55: How many calories are required to raise
Q56: The specific heat of a substance is
Q57: Raising the temperature of 10.0 g of
Q58: The number of calories needed to raise
Q60: The heat of fusion for water is
Q61: The amount of heat necessary for one
Q62: A temperature of 50.°F is equivalent to
Q63: A 12-g sample of cooking oil,which is
Q64: The change of state from solid to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents