According to the Brønsted-Lowry definition,________.
A) an acid is a proton acceptor
B) a base produces 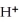 ions in aqueous solutions
ions in aqueous solutions
C) a base is a proton donor
D) a base is a proton acceptor
E) an acid acts as the solvent
Correct Answer:
Verified
Q5: Identify the Brønsted-Lowry acid in the following
Q6: The name given to an aqueous solution
Q7: Which of the following is correctly identified?
A)
Q8: The name of Q9: Which of the following statements correctly describes Q11: According to LeChâtelier's principle,predict whether adding Q12: Which of the following is the strongest Q13: The correct formula for sulfuric acid is Q14: When a reaction is at equilibrium,_. Q15: The conjugate base of ![]()
A)all reaction![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents