What is the function of 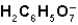 in the first ionization of citric acid?
in the first ionization of citric acid? 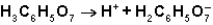
A) The ion serves as an Arrhenius acid in this reaction.
B) The ion serves as an Arrhenius base in this reaction.
C) The ion serves as the conjugate base of the acid,H3C6H5O7.
D) The ion serves as the conjugate acid of the base,H3C6H5O7.
Correct Answer:
Verified
Q1: According to the Arrhenius theory,what is produced
Q2: A water solution is found to have
Q3: Identify the Brønsted acid(s)in the reaction. HIO3(aq)+
Q5: The molar concentration of H+ ions in
Q6: Which of the following substances could behave
Q6: Which of the following statements is true
Q7: The cation (positive ion)in a salt comes
Q11: What are the missing products in
Q14: In the Brønsted theory,both acids and bases
Q16: Which of the following is present in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents