Phosphoric acid,H3PO4,undergoes three dissociation reactions.Which of the three acids is the weakest?
A) 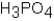
B) 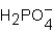
C) 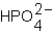
D) More than one response is correct.
Correct Answer:
Verified
Q21: How many equivalents are contained in 0.25
Q27: Which of the following is a weak
Q28: Which of the following mixtures would represent
Q30: If a solution of acetic acid (a
Q33: A solution for which [OH-] = 3.0
Q33: Which of the following salts would produce
Q38: In a buffer solution made up of
Q41: The expression for the Ka of the
Q50: A reaction in which an acid and
Q55: The buffer capacity is the amount of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents