The dissociation reaction for the weak acid,H2PO3- would be which of the following?
A) 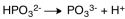
B) 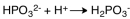
C) 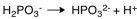
D) 
Correct Answer:
Verified
Q41: Which of the following sets of reactants
Q42: A patient comes to you suffering from
Q44: HCl will react with
A)BaO
B)CaCO3
C)Mg
D)All three are correct.
Q44: To determine the number of equivalents of
Q46: Identify two Brønsted base(s)in the reaction. HIO3
Q49: When your liver detoxifies ethyl alcohol, the
Q49: How many meq are contained in 25.00
Q50: What is the pKa of an acid
Q54: Which of the following would you expect
Q60: A 10-8 M solution of HCl is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents