The following reaction is observed in a lab experiment: A + 2B C + D In this experiment,it required 750 s for the concentration of C to change from 0.333 M to 0.750 M.What is the rate of the reaction?
A) 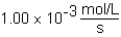
B) 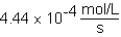
C) 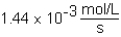
D) 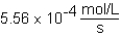
Correct Answer:
Verified
Q43: The device used to measure heat produced
Q44: Which of the following is NOT true
Q53: An elderly person comes to you on
Q56: Consider the following energy diagram.Which letter represents
Q58: If we remove CO2 from the following
Q60: Which of the following would be the
Q62: Catalysts may lower the activation energy.
Q69: Reactions that have a low energy of
Q70: Increasing the concentration of a reactant will
Q79: A reaction rate can be described in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents