The reaction below does not progress at room temperature,but does at 1100 C.Therefore,this reaction is spontaneous at 1100 C. 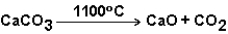
Correct Answer:
Verified
Q60: Which of the following would be the
Q62: Catalysts may lower the activation energy.
Q63: Butane, C4H10, burning in air, is an
Q67: The effect of a catalyst is to
Q69: Reactions that have a low energy of
Q70: Increasing the concentration of a reactant will
Q71: A spontaneous process accompanied by an entropy
Q74: Chemical reactions always occur once the molecules
Q75: A reaction will occur each time two
Q79: A reaction rate can be described in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents