A solution is prepared at 75 °C by dissolving 50.0 g of A in 100 g of water.When this solution is cooled to 15 °C what happens based on the following solubility plot for A. 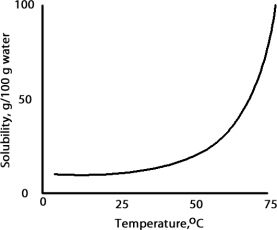
A) Solid A crystallizes and the solution is unsaturated.
B) Solid A dissolves and the solution is supersaturated.
C) Solid A crystallizes and the solution is saturated.
D) Solid A dissolves and the solution is unsaturated.
Correct Answer:
Verified
Q45: Express the following concentration of solution in
Q46: When sodium acetate dissolves in water,the following
Q46: You discover that your roommate has left
Q50: Which of the following correctly arranges 1.00
Q51: A solution is prepared at 75 °C
Q52: Citric acid,a natural food preservative,accounts for the
Q53: Which of the following would be considered
Q57: Drinking water can be purified by which
Q58: An ion in solution that is surrounded
Q66: Polar substances tend to dissolve in non-polar
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents