In which of the following does an element have an oxidation number of +4?
A) TiO2
B) HBr
C) SO3
D) 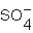
Correct Answer:
Verified
Q2: Single replacement reactions are always
A)redox reactions.
B)nonredox reactions.
C)combination
Q3: What is the oxidation number of Cl
Q8: Which species would be considered a
Q14: Which set of coefficients balances the
Q14: Which of the following is an oxidizing
Q15: Which of the following statements is
Q16: When the equation below is properly
Q17: Which of the following equations (not
Q18: Which of the following reactions is
Q18: A REDOX reaction can also be a(n)
A)combination
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents