When the following hydrocarbon reacts with an excess of oxygen gas,the balanced equation would contain how many moles of water? 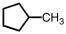
A) 5
B) 6
C) 10
D) 12
Correct Answer:
Verified
Q24: Alkanes are _ in water and _
Q32: An alkane with 10 carbon atoms is
Q34: How many isomers (structural + geometric)exist for
Q39: The correct name for the alkyl group
Q41: The structural formula of 1-bromo-2,2-dimethylbutane is _
Q45: Alkanes react readily with _ .
A)sodium hydroxide
B)hydrochloric
Q46: When ethane burns in a limited supply
Q47: What is the IUPAC name for the
Q51: You arrive home from class to find
Q56: How many hydrogen atoms are needed to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents