Sample #1 is made from an isotope with decay constant 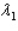 and sample #2 is made from an isotope with decay constant
and sample #2 is made from an isotope with decay constant 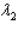 ,where
,where 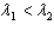 .Which of the following statements must be true?
.Which of the following statements must be true?
A) The activity of sample #1 is greater than that of sample #2.
B) The activity of sample #2 is greater than that of sample #1.
C) The half-life exhibited for sample #1 is greater than that of sample #2.
D) The half-life exhibited for sample #2 is greater than that for sample #1
Correct Answer:
Verified
Q62: The Q value of the reaction
Q63: Which of the following is not true
Q63: The isotope 238U,which starts one of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents