For the n = 4 shell,what are the lowest values possible for 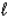 and
and 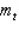 respectively?
respectively?
A) 0,0
B) -4,-4
C) 0,-3
D) -3,-3
Correct Answer:
Verified
Q32: Consider the hydrogen atom,singly ionized helium atom,and
Q49: The quantity _,where Ψ is the wave
Q59: How many electrons are in bromine's (atomic
Q61: In the n = 4 shell,how many
Q62: The red light from a HeNe laboratory
Q63: If a hydrogen atom,originally in its ground
Q67: The stimulated emission of photons from the
Q68: The wavelength of coherent ruby laser light
Q69: In neon,the 20.66-eV level can undergo lasing
Q72: Which of the following conditions must be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents