An ideal gas at pressure,volume,and temperature,P0,V0,and T0,respectively,is heated to point A,allowed to expand to point B also at A's temperature 2T0,and then returned to the original condition.The internal energy decreases by 3P0V0/2 going from point B to point T0.How much heat left the gas from point B to point T0? 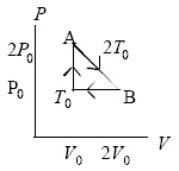
A) 0
B) P0V0/2
C) 3P0V0/2
D) 5P0V0/2
Correct Answer:
Verified
Q5: Which of the following increases the internal
Q18: Heat is applied to an ice-water mixture
Q25: A turbine takes in 1 000-K steam
Q27: As the ideal gas expands from pressure
Q28: An ideal gas at pressure,volume,and temperature,P0,V0,and T0,respectively,is
Q29: A cylinder containing an ideal gas has
Q31: How much thermal energy must be added
Q32: A heat engine operating between a pair
Q33: An electrical generating plant operates at
Q34: System 1 is 3.5 moles of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents