Use Bohr's model to draw a sodium (Na) atom and a chlorine (Cl) atom. Using your model, explain what happens when sodium reacts with chlorine to form table salt. Include in your explanation ion and ionic bond formation. Use your model to help you to decide whether NaCl is hydrophilic or hydrophobic. 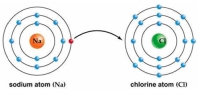
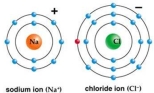
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q46: Which one is NOT one of the
Q47: Which substances are on the basic side
Q48: What is the maximum number of electrons
Q49: The electrons are unequally shared in _,
Q50: How many atoms are required to form
Q52: All living things are 70 - 90%
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents