Suppose 100 mL of a 0.2 M aqueous solution of histidine is titrated with 0.4 M NaOH.Given that the pKa of the -COOH group,the -NH3+ group,and the -R group are approximately 1.80,9.33,and 6.04,respectively,sketch the titration curve. 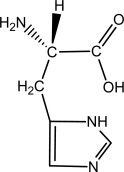 histidine
histidine
What are the approximate pKa values of the carboxylic acid group,the amine group,and the side group (if applicable)?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q62: How many different nucleotides are used to
Q101: Which of the following statements regarding transcription
Q117: Region II in the nucleotide shown is
Q118: Region I in the nucleotide shown is
Q119: Which of the following is NOT part
Q120: Which of the following statements regarding the
Q121: Use the Henderson-Hasselbalch equation to explain why
Q125: RNA contains the sugar _ and the
Q138: Two dipeptides can be produced from the
Q154: Describe the structural features of an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents