Alcohols used as gasoline additives or substitutes are often called biofuels.Similar organic compounds derived from vegetable oils and fats are called biodiesels,which are another class of organic chemicals called esters.Qualitatively compare the combustion energy content (kJ/g) of the four-carbon alcohol,butanol,with the energy content (kJ/g) of the four-carbon ester,ethyl acetate. 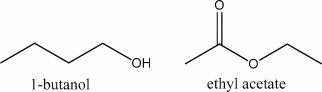
A) Ethyl acetate has less energy/mass than butanol.
B) Ethyl acetate has more energy/mass than butanol.
C) The two compounds have roughly the same energy/mass.
D) These are not two different compounds and so must have the same energy/mass.
E) There is no basis for determining this without some form of enthalpy values.
Correct Answer:
Verified
Q79: Which of the following class of compounds
Q85: Which of the following statements regarding methanol
Q102: Which of the following statements regarding methanogenic
Q105: What is the by-product of the preparation
Q114: Which of the following statements regarding diethyl
Q116: Which one of the following reactions
Q118: MTBE (methyl tert-butyl ether)is: Q120: Ethanol is a renewable chemical energy source,but Q122: Which of the following pairs of molecules Q123: Which of the following has an amide
A) ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents