A voltaic cell is constructed based on the oxidation of zinc metal and the reduction of silver (I) ions.Solutions of 1.00 M silver nitrate and zinc nitrate also were used.The anode is on the left; the cathode,on the right.Where does oxidation occur? 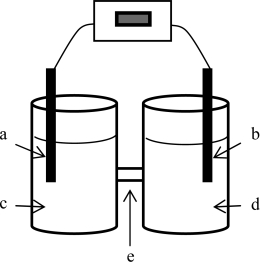
A) a
B) b
C) c
D) d
E) e
Correct Answer:
Verified
Q3: Reduction refers to
A)loss of mass.
B)an increase in
Q16: Consider the reaction,Sn + Au3+
Q17: In the smelting of iron from
Q19: When hydrogen reacts with a metal to
Q20: The following reaction occurs in acidic
Q22: Balance the chemical equation for the
Q23: Based on the cell diagram,Fe(s)| Fe2+(aq)|| O2(g)|
Q24: Balance the chemical equation for the
Q25: A voltaic cell is constructed based on
Q26: Which statement about a cathode in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents