A voltaic cell is constructed based on the reaction of Br -(aq) with Cl2(g) producing Cl -(g) and Br2( 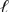 ) ,with platinum serving as inert electrodes.Identify the correct cell diagram.
) ,with platinum serving as inert electrodes.Identify the correct cell diagram.
A) Br2( 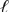 ) | Br-(aq) || Cl2(g) | Cl-(aq)
) | Br-(aq) || Cl2(g) | Cl-(aq)
B) Pt(s) | Cl2(g) | Cl-(aq) || Br2( 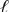 ) | Br -(aq) | Pt(s)
) | Br -(aq) | Pt(s)
C) Pt(s) | Br--(aq) | Cl2(g) || Br2( 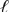 ) | Cl-(aq) | Pt(s)
) | Cl-(aq) | Pt(s)
D) Pt(s) | Br -(aq) | Br2( 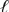 ) || Cl2(g) | Cl-(aq) | Pt(s)
) || Cl2(g) | Cl-(aq) | Pt(s)
E) Pt(s) | Br2( 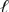 ) | Br -(aq) || Cl2(g) | Cl-(aq) | Pt(s)
) | Br -(aq) || Cl2(g) | Cl-(aq) | Pt(s)
Correct Answer:
Verified
Q24: Which one of the following items does
Q31: A voltaic cell is constructed based on
Q32: A voltaic cell is constructed based on
Q33: Balance the chemical equation for the
Q34: A voltaic cell is constructed based on
Q35: Which of the following statements regarding reduction
Q37: Balance the chemical equation for the
Q38: Which statement about a voltaic cell is
Q39: Balance the chemical equation for the
Q40: A voltaic cell is constructed based on
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents