Silver tarnish (Ag2S) can be removed by immersing silverware in a hot solution of baking soda (NaHCO3) in a pan lined with aluminum foil; however,foul-smelling hydrogen sulfide gas (H2S) is produced.What is 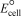 for the redox reaction that occurs?
for the redox reaction that occurs? 
A) +3.512 V
B) +1.051 V
C) +2.461 V
D) +1.521 V
E) +0.940 V
Correct Answer:
Verified
Q44: The standard hydrogen electrode is
A)used to calibrate
Q53: The change in free energy for
Q62: The magnitude of the charge on
Q66: The work involved in moving exactly 1
Q74: An electrochemical cell is constructed with
Q76: An electrochemical cell contains a standard hydrogen
Q79: Which of the following statements regarding chemical
Q81: Consider the voltaic cell based on
Q82: Neuron cells generate electrical signals by concentration
Q83: Which statement does NOT correctly describe a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents