A concentration cell is constructed by using the same half-reaction for both the cathode and anode.What is 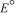 for a concentration cell that combines silver electrodes in contact with 0.10 M silver nitrate and 0.00003 M silver nitrate solutions? (
for a concentration cell that combines silver electrodes in contact with 0.10 M silver nitrate and 0.00003 M silver nitrate solutions? ( 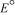 = +0.80 V for Ag/Ag+)
= +0.80 V for Ag/Ag+)
A) "+0.21 V"
B) "+0.59 V"
C) +0.80 V
D) "-0.21 V"
E) "+1.01 V"
Correct Answer:
Verified
Q81: Consider the voltaic cell based on
Q82: Neuron cells generate electrical signals by concentration
Q83: Which statement does NOT correctly describe a
Q84: Calculate the equilibrium constant for the
Q85: An electrochemical cell is constructed with
Q87: A concentration cell with a cell
Q88: A pH meter uses an electrode arrangement
Q89: What is the cell potential of
Q89: An electrochemical cell has both a silver
Q90: A concentration cell is constructed by using
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents